| 32nd Annual
ISO 9000 & AUDITS WORLD CONFERENCE |
|
 |
|
 |
|
Conference Registration is open for Conferees, Speakers and Exhibitors Download a copy of the ISO Conference at-a-Glance WHOVA | SPEAKERS  Speakers are encouraged to use the official ISO Conference template. Please download here. Speakers' Meeting: | EXHIBITS March 18-19, 2024 Setup: Exhibit Hours: Teardown: Tuesday 3/19, 2024: | SOCIAL HOUR  Hawaiian Luau Party Monday, March 18, 2024; 5:30 PM - 6:30 PM Aloha! Let’s open up ISO 9000 Social Hour 2024 Hawaiian style! Join us for networking and happy hour! Wear your loudest tropical shirt or festive Luau gear for networking fun! Mahalo! |
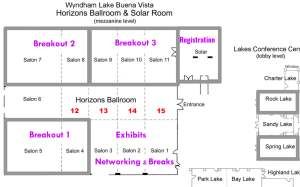
REGISTRATION - Solar Foyer SUN March 17; 8 AM-9 AM & 4 PM-5 PM MON March 18; 7:30 AM-9 AM & 4 PM-5 PM TUE March 19; 7:30 AM-9 AM & 4 PM-5 PM Gift Distribution: International conferees can pick up their early registration gifts during the registration hours. For U.S. conferees, early registration gifts will be shipped by March 31st. Floor Layout (click on the link to see the large map) | pre-conference Workshop 1Sunday, March 17, 2024 Understanding ISO 9001 to
Lorri Hunt, President, Lorri Hunt & Associates, Inc., Kansas City, MO, USA 12 PM- 1 PM: Lunch Break - Sandy Lake Room | pre-conference Workshop 2Sunday, March 17, 2024 Understanding the New AS9100 and IA9100 Aerospace Standards Requirements
Buddy L. Cressionnie, IAQG-1 Standard Vice Chair and Americas IA9100 Leader Alan W. Daniels, Quality Senior Management, The Boeing Company, Lake Stevens, WA, USA 12 PM- 1 PM: Lunch Break - Sandy Lake Room |
CONFERENCE AGENDA
Quality in the AI Era
MARCH 18, 2024 - MONDAY MORNING
10:15 AM–10:45 AM; Coffee Break; Horizon Ballroom 1-3
12:00 PM -1:15 PM; Lunch Break; The Lakeview Restaurant |
MARCH 18, 2024 - MONDAY AFTERNOON
|
|
|
3:00 PM–3:20 PM; Coffee Break; Horizon Ballroom 1-3
CHAMPAGNE NETWORKING | New to the conference this year, attendees will have the opportunity to network with the conference speakers and interact with fellow attendees at the same time. The speaker will be facilitating a table topic discussion in a group setting providing an excellent opportunity for knowledge exchange. Attendees will get a chance to sign up for up to four table topics using the online registration form. Speakers will be positioned at tables around the room. To start the event, attendees will join the first table topic they signed up for. After a brief introduction, the speaker will facilitate a conversation and lead a discussion on their topic. After a 20-minute round, then it’s on to the next table for a total of four rounds! Best of all, discussions can be continued throughout the conference! |
3:20 PM–4:40 PM Horizon Ballroom 1-3 |
Creating, Using, and Auditing FMEAs Patsy L. Brown, Consultant, Trainer, Brown & Associates Quality Consulting, Inc., Pine Bluff, AR, USA
|
How Do You Use Games and Simulations to Make Learning “Stick”? Kevin Clay, President & CEO, Six Sigma Development Solutions, Inc., Pensacola Beach, FL, USA |
Climate Change & ISO 9001 Robert Freeman, Managing Director, ACI Assurance, LLC, Dallas, TX, USA
|
Value Creation, a Role for Quality Professionals Gautam Mahajan, President, Customer Value Foundation, New Delhi, IN-DL, India
|  Engaging Employees in Your QMS Kathy Abbett, Consultant, Abbett Consulting, Glen Carbon, IL, USA |
Earth to Earth Sustainable Supply Chains Michael Ford, Principal, TQM Works Consulting, Port Crane, NY, USA |
MARCH 19, 2024 - TUESDAY MORNING |
|
|
|
9:55 AM–10:15 AM; Coffee Break; Horizon Ballroom 1-3
|
|
|
12:00 PM -1;15 PM; Lunch Break; The Lakeview Restaurant
MARCH 19, 2024 - TUESDAY AFTERNOON
|
|
3:00 PM–3:15PM; Coffee Break; Horizon Ballroom 1-3
|
|
|